All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, and Syndax, and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Risk stratification using FLT3 and NPM1 in patients with acute myeloid leukemia autografted in first complete remission — A retrospective, multicenter analysis study
Autologous stem cell transplantation (auto-SCT) has been reported to be an effective therapy against acute myeloid leukemia (AML), especially in patients with favorable cytogenetic risk. Although survival with auto-SCT may surpass other post-remission therapies (PRTs), the major concern is relapse, since it does not have a graft-versus-leukemia effect. Data on how specific combinations of some markers (FLT3-ITD and NPM1) affect prognosis in autografted patients with intermediate-risk cytogenetics, are still lacking.
Roni Shouval and colleagues assessed the prognostic role of FLT3-ITD and NPM1 in patients who underwent auto-SCT, and reported the results to the European Society for Blood and Marrow Transplantation (EBMT) registry. The Acute Leukemia Working Party (ALWP), of the EBMT registry, is a voluntary working group reporting all consecutive stem cell transplantations and follow-ups once a year. Shouval et al. published the study1 in Bone Marrow Transplantation, and concluded that patients with AML having intermediate-risk cytogenetics and FLT3-ITDneg/NPM1mut have favorable outcomes when autografted in first complete remission (CR1), suggesting that auto-SCT is a valid PRT option.
Study design
This was a retrospective, multicenter analysis of more than 600 transplantation centers, covering the period from Jan 1, 2000 to Dec 31, 2014. All patients participating in this study provided written informed consent, indicating their approval of the use of information for research purposes. Adult patients with de novo AML were required to be in CR1 and classified as possessing intermediate-risk cytogenetics according to Medical Research Council classification. Available data on FLT3-ITD and NPM1 were mandatory to meet the inclusion criteria for this study.
Patients were classified into one of the following molecular subtypes of AML: FLT3-ITDneg/NPM1mut, FLT3-ITDpos/NPM1mut, FLT3-ITDneg/NPM1WT, and FLT3- ITDpos/NPM1WT. Data on AML treatment prior to auto-SCT are not captured in the registry.
The primary outcome was leukemia-free survival (LFS), which is defined as survival without leukemia progression or relapse, following auto-SCT. The secondary outcomes were overall survival (OS), transplantation-related mortality (defined as death without previous relapse/progression), and relapse (defined as recurrence of leukemia at any site). All outcomes were measured from the time of auto-SCT.
Clinical, demographic, treatment, and outcome data were summarized and performed via median values with interquartile ranges or percentages. Comparison of baseline features between patients with the different AML molecular subgroups were carried out through the Mann-Whitney U test or the Chi-square test. The probabilities of LFS and OS were calculated using the Kaplan-Meier method. The transplantation-related mortality, and relapse incidence (RI) were calculated by cumulative incidence using the Fine-Gray method, considering the competition between these two events.
Results
- The median age of patients was 53, and the majority had good Karnofsky performance status at the time of transplantation
- FLT3-ITDneg/NPM1WT was the leading molecular category (50%), followed by FLT3-ITDneg/NPM1mut (30%), FLT3-ITDpos/NPM1mut (11%), and FLT3-ITDpos/NPM1WT (9%)
- The majority of patients had AML with normal karyotype (84%)
- Patients were transplanted at a median duration of 4.95 months from the diagnosis of AML
- The graft source was primarily mobilized peripheral blood (93%)
- Cyclophosphamide and busulfan were the leading preparative regimen (47%), followed by busulfan and melphalan (25%)
- 10% of patients received total body irradiation (TBI) as part of conditioning
- Median doses (interquartile ranges) of conditioning components were 120 mg/kg (120–210) for cyclophosphamide, 140 mg/m2 (140–140) for melphalan, 12 Gy (12–12) for TBI, 12.8 mg/kg (10.1–12.8) for intravenous busulfan, and 16 mg/kg (16–16) for oral busulfan
FLT3-ITD and NPM1 mutations could refine the prognostic stratification in AML with intermediate-risk cytogenetics. Yet, data on their role in patients undergoing auto-SCT as PRT are scarce. In this study, researchers retrospectively evaluated the role of FLT3-ITD and NPM1 in a cohort of AML patients (n = 405) with intermediate-risk cytogenetics, autografted in CR1, over a median follow-up of 5.5 years.
Fig. 1 Univariate analysis of 5-year survival and recurrence by molecular subtype. FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication; NPM1, nucleophosmin-1; LFS, leukemia-free survival; OS, overall survival; RI, relapse incidence
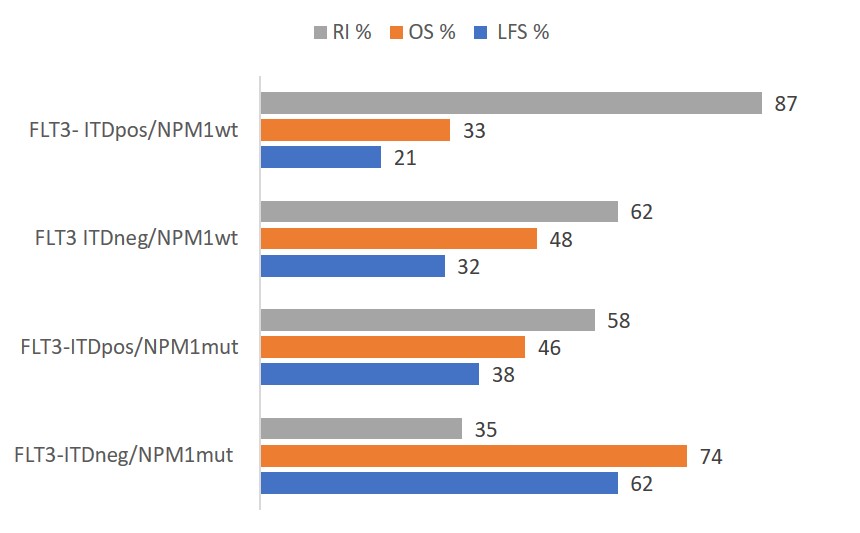
The 5-year LFS was the highest for patients with FLT3-ITDneg/NPM1mut (62%), and the lowest for patients with FLT3- ITDpos/NPM1WT (21%). At 5 years, OS and RI were also favorable in patients with the FLT3-ITDneg/NPM1mut subtype, at 74% and 35%, respectively, compared with other subtypes (see Fig. 1). In the univariate analysis, molecular subtype was correlated with LFS (5-year LFS, 62%; p < 0.001), OS (5-year OS, 74%; p < 0.001), and RI . The molecular subtype was the strongest predictor of LFS, OS, and relapse in a Cox multivariable model adjusted for sex, age, transplantation year, time from diagnosis to transplantation, graft source, and TBI administration.
A quarter of the patients (n = 103) had a subsequent allogeneic stem cell transplantation (allo-SCT). The distribution via genetic profile of allo-SCT were 18%, 24%, 29%, and 32% for
FLT3-ITDneg/NPM1mut, FLT3-ITDpos/NPM1mut, FLT3-ITDneg/NPM1WT, and FLT3-ITDpos/NPM1WT, respectively (p = 0.093).
Conclusion
In conclusion, CR1, FLT3, and NPM1 were the most influential determinants of outcomes in AML patients with intermediate-risk cytogenetics, who underwent auto-SCT. This study indicates that auto-SCT should be avoided in patients with high-risk AML (FLT3-ITDpos/NPM1WT) as relapse rates are extremely high. For those patients without FLT3-ITD, survival following transplantation in the NPM1mut AML subtype is favourable, indicating that allo-SCT could be a possibility for salvage treatment in CR2 patients. Within the intermediate-risk group (FLT3-ITDneg/NPM1WT and FLT3-ITDpos/NPM1mut), auto-SCT did not provide adequate disease control but may be considered an option in populations where allo-SCT is contra-indicated (e.g., elderly and frail). In summary, the combination of novel targeted therapy with patient selection based on MRD status, could improve patient outcomes following auto-SCT.
Find out more about the use of FLT3/ITD status as a prognostic marker in the following articles:
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

